“Bunker busting” Antibody-antibiotic-conjugates (AACs) successfully used to target MRSA bacteria hiding inside the host’s cells
One of the difficulties in combating MRSA is that (Staphylococcus aureus) bacteria have the ability to live inside the host’s cells where they are effectively sheltered from the action of systemic antibiotics – it is this reservoir of infection that provides the seed for the relapses that are characteristic of MRSA.
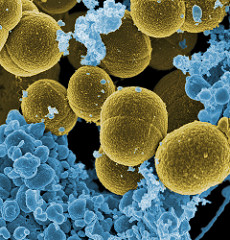
S. aureus bacteria escaping a white blood cell, x20,000 mag.
Credit: NIAID – Creative Commons CCBY2
A large team of researchers from Genentech in the USA and Symphogen in Denmark has developed a method to destroy these intracellular S.aureus bacteria that would otherwise be protected from antibiotics. The team used a novel conjugate of the antibiotic rifalogue together with monoclonal antibodies. These antibody-antibiotic-conjugates (AACs) are specifically immuno-targeted to S.aureus. The AACs remain inactive in the bloodstream, and only become active in the presence of the bacteria inside the host´s cells.
Once the AACs are taken into the host’s cells they are transported deeper – into the phagolysosomes which enclose the bacteria inside the cell. The phagolysosomes contain a proteolytic environment, and the protease enzymes found there cleave a small peptide group from the AACs – activating them. The active AACs are then able to bind to the surface of the bacteria effectively delivering their antibiotic payload to the infection’s hidden “bunker”.
Infected mice were treated with the AACs, and the team found this new treatment was much more effective than a systemic antibiotic (vancomycin). This work confirms the importance of intracellular S.aureus as a reservoir of MRSA infection, and raises the exciting possibility that these AACs might eventually be used to treat humans. The targeted approach of AACs would avoid damaging the patient’s beneficial microflora, and if their use becomes routine it would probably reduce the rate at which bacteria in general evolve resistance to any one particular antibiotic.
(This study was published in Nature:
Sophie M. Lehar et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus, Nature (2015). DOI: 10.1038/nature16057 http://www.nature.com/nature/journal/v527/n7578/full/nature16057.html )